Español 简体中文
Are you or a loved one having a hard time hearing? Perhaps you’re thinking about getting a hearing aid.
Hearing aid technology keeps evolving, which means there’s a growing variety of styles & features to consider.
“People who already use a hearing aid know that selecting one is not a simple decision,” says Eric Mann, M.D., Ph.D., chief medical officer in the FDA office responsible for hearing aids. “Hearing loss affects people in different ways. So, it’s important to choose a hearing aid that’s appropriate for your condition & fits your lifestyle.”
The U.S. Food & Drug Administration regulates hearing aids to make sure they are safe & effective. If you’re considering hearing aids, this article highlights some common technologies & terms you may encounter & notes a change that’s coming to how hearing aids are sold.
Hearing Aids & How They Work
People may be born with hearing loss. Or they may develop it later in life—often because the inner ear can wear out as we age or be damaged by years of exposure to loud noises.
In some cases, hearing loss is temporary & can be restored with medical help. In other cases, it’s permanent but can be improved with hearing aids.
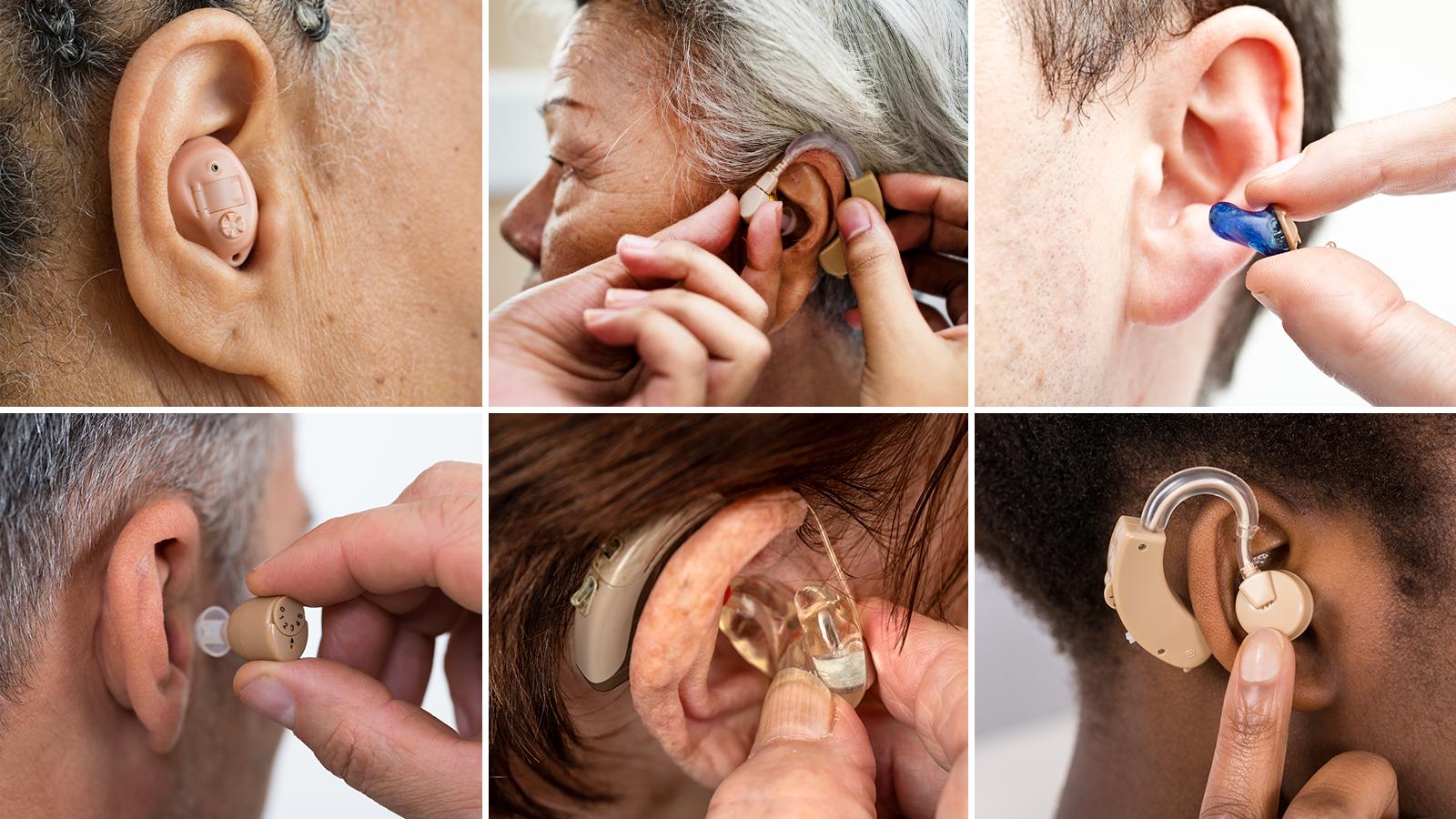
Hearing aids are medical devices worn behind or in the ear. They can improve hearing by making sounds louder. However, hearing aids usually won’t restore your hearing to normal levels or quality in the way that eyeglasses can often restore vision to 20/20.
Air-conduction vs. bone-conduction hearing aids
Most hearing aids work through air conduction. They bring amplified sound into the ear canal. Sound then moves through the eardrum & three tiny bones in the middle ear to reach the inner ear, where it’s processed & sent to the brain.
For people who have problems with their outer or middle ear, those areas can be bypassed with bone-conduction hearing aids. They send sound through the skull to reach the inner ear.
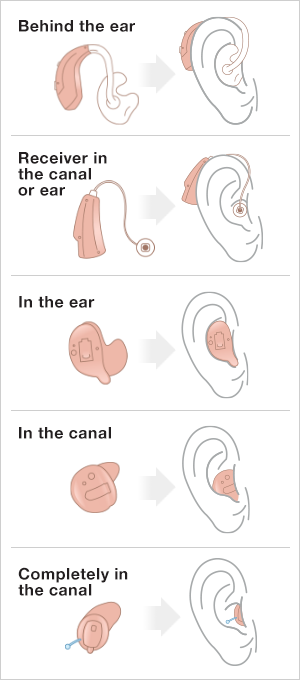
Styles of Hearing Aids
Behind-the-ear (BTE) aids: BTE hearing aids are generally the largest hearing aid style. A plastic case containing most of the electronics sits behind the ear & is connected to an earmold that fits in the ear canal. BTE hearing aids can be used by people of all ages. The style is often chosen for young children because it can be adapted as they grow.
Receiver-in-the-canal (RIC) aids: RIC (or mini receiver-in-the-ear; mini RITE) hearing aids sit behind the ear but are typically smaller than a BTE. The RIC hearing aid is attached to a tube housing a small wire with a dome-shaped tip at the end that rests in the ear canal (in some cases, earmolds are used). The RIC design allows more of the ear canal to remain open & is less visible than the BTE style.
In-the-ear (ITE) aids: This hearing aid sits completely in the outer ear (the “bowl” of the ear). All the hearing aid electronics are housed in a custom-fit shell.
In-the-canal (ITC) aids & completely-in-the-canal (CIC) aids: These are the smallest hearing aids currently available. The electronics are contained in a small custom-fit shell that fits partly or completely into the ear canal. Some people may like them because they are less noticeable while other people may find them harder to handle.
Hearing Aid Features
Today’s hearing aids come with a variety of features. Here are some of the more common ones.
Directional microphones focus on sound from a specific direction. They could help you hear someone in a face-to-face conversation over the noise around you, for example.
Telecoils enable the hearing aid to pick up sound directly from compatible phones or compatible sound systems in public places, such as theaters & houses of worship.
Wireless connectivity such as Bluetooth allows hearing aids to interact with televisions, cellphones, computers or tablets, for example.
Getting Hearing Aids
Medical evaluation required for children (younger than 18 years of age)
While hearing loss in adults is often caused by aging or noise exposure, the reasons for hearing loss in children are more varied & may be associated with other medical conditions requiring medical evaluation & treatment. So, the FDA requires a statement of a doctor’s exam before the sale of hearing aids for children.
The FDA does not intend to enforce the requirement that people 18 years of age & older have a medical evaluation statement (or sign a waiver) before the sale of certain hearing aids.
Hearing aids typically are sold by audiologists; ear, nose & throat doctors; or sellers licensed to dispense hearing aids, such as instrument specialists.
Proposed over-the-counter (OTC) hearing aids
Some hearing aids can be legally sold directly to the user over the internet or through mail order if permitted in your state.
To broaden access to hearing aids, the FDA is proposing a new category of over-the-counter (OTC) hearing aids that you could buy in the store or online without seeing a physician for an exam or an audiologist for help with fitting. After the new FDA regulations are finalized, hearing aids could become more widely available nationwide.
The proposed OTC rules would apply to certain air-conduction hearing aids intended for people age 18 & older who have perceived mild to moderate hearing loss. A person with mild hearing loss may hear some speech sounds but not others. A person with moderate hearing loss may hear almost no speech when someone is talking at a normal level.
“We want hearing aids to be more readily available & accessible, especially as our population ages,” Mann explains. “It’s also important for people to recognize that hearing loss could be a sign of an easily treatable problem like built-up earwax or a more serious problem like a benign tumor on the hearing nerve. See a doctor when things don’t feel right, when your hearing loss is progressing, or if you are having associated symptoms like dizziness, ear pain, or drainage from the ear canal.”
Hearing aids vs. personal sound amplification products
You may have seen products in stores or online that are known as personal sound amplification products (PSAPs). These are not alternatives to hearing aids.
While hearing aids & PSAPs both amplify sound for the user, the products have different intended uses. Hearing aids are intended to make up for impaired hearing. PSAPs, in contrast, are intended for people with normal hearing to amplify sounds in certain situations, such as recreational activities like birdwatching or hunting.
Because such PSAPs are regulated as consumer electronics & not medical devices, they may be more variable in terms of product quality compared to hearing aids. The FDA does not regulate such PSAPs for safety & effectiveness like we do for hearing aids.
Additional Resources
- FDA webpage, Hearing Aids
- FDA Consumer Update, Cochlear Implants: A Different Kind of ‘Hearing’
- National Institute on Deafness & Other Communications Disorders, Hearing, Ear Infections & Deafness
- Immediately in Effect Guidance Document: Conditions for Sale for Air-Conduction Hearing Aids
- Proposed Rule: Medical Devices; Ear, Nose, & Throat Devices; Establishing Over-the-Counter Hearing Aids
- Draft Guidance: Regulatory Requirements for Hearing Aid Devices & Personal Sound Amplification Products
Source: FDA Consumer Updates